*Archived* ASCEND Osteoarthritis Knee Pain Study
*Archived*
Study No Longer Active
Now Taking Applications
Is osteoarthritis knee pain keeping you from performing daily activities? If you are tired of short-term solutions to treat your knee pain, consider joining the ASCEND study, a clinical trial for osteoarthritis of the knee patients studying an investigational OA knee treatment.
ASCEND OA Knee Pain Study
Help us test an investigational study drug for Osteoarthritis Knee Pain.
What is the ASCEND study?
The ASCEND clinical research study is for people who have painful osteoarthritis of the knee (OA). The study will testan investigational OA knee treatment to learn if it can potentially provide long-lasting relief from pain and slow down its progression.

You may be able to join the ASCEND Osteoarthritis Knee Pain study if you:
- Are 45 to 80 years old
- Confirmed diagnosis of osteoarthritis of the knee
- Experience consistent knee pain (every other day or more on average) for the past month.
- Have tried at least 2 therapies for your knee pain, such as physical therapy or NSAIDs. NSAIDs are a type of over-the-counter pain medication, such as ibuprofen (Advil®) or naproxen (Aleve®)
- Other criteria apply. Ask your doctor to help you find out if you may qualify
May receive compensation up to $3,150 for inconvenience and travel.

Eligible participants receive the following as part of the study:
- Study drug or placebo (no active ingredients).
- Study-related health tests.
- Study-related support and monitoring by a healthcare team.
- Information about osteoarthritis of the knee.
Complete The Questionnaire Below To Submit Your Information For Future Studies

About Osteoarthritis of the Knee
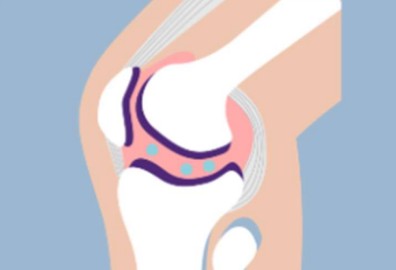
What is osteoarthritis of the knee?
Knee osteoarthritis (OAK) is when the cushion between the bones, called cartilage, wears down over time. Without enough cushion, the bones start to rub against each other, which can cause knee joint pain/tenderness, decreased function, stiffness, and swelling.
At this time, there is no progression-modifying treatment available for OA of the knee and its associated pain. Some subjects may live for decades with the disease. People living with OA of the knee may turn to pain relief options such as supplements, physical therapy, or knee replacement surgery.
The goal of the ASCEND study is to provide a potential solution for patients suffering from this condition that may be long-lasting, slows down progression, and doesn’t require surgery.
Where can I learn more?
- Osteoarthritis of the Knee | Arthritis Foundation
- Osteoarthritis – Diagnosis & treatment – Mayo Clinic
- Arthritis in Knee: Symptoms, Causes and Treatment

Diversity in Clinical Research Studies
People may experience respiratory diseases in different ways. We plan to include people of different races, ethnicities, genders, ages, and backgrounds in our OA Knee Pain Study. This will help us to see how the research being studied works for different people.
Why are clinical studies important?
Clinical trials are the best way to find out if new treatments or vaccines work and how safe they are. If clinical trials show that a new treatment works and is safe, then it can be approved to be used by the people who need it.
Not all clinical trials test a new treatment or vaccine. “Observational” studies collect information about people’s health during their normal care. This helps researchers learn more about specific health issues.

What is a clinical research study?
A clinical research study is a medical study that helps to answer important questions about an investigational medication or vaccine. such as:
- Is it effective?
- What amount. or dose. may work best?
- How safe is it?
- Are there side effects?
All medications and vaccines must be tested in clinical research studies before they can be approved for use. Without people taking part in these studies, we would have no new medications or vaccines
Clinical research studies should include groups of people who may be at higher risk based on their age, sex. and race/ethnicity.
An Institutional Review Board (IRB)/Ethics Committee (EC), which protects the rights, safety, and wellbeing of the participants has approved this study.


